

| Limescale - Prevention and Dissolution | |||||
 |
 |
 |
 |
||
|
Rainwater is slightly acidic, i.e. soft. In heavily industrialized regions, emissions make the rainwater more acidic. The hardness in water comes from the calcium and magnesium salts, that are dissolved into the water, from soluble rocks through which the rainwater flows. Hard water contains both temporary and permanent hardness. Temporary hardness in most cases is associated with calcium and magnesium carbonates and bicarbonates. These crystal forming salts are held in solution and will remain so, unless there is a change in pressure or temperature, which will cause the water to become supersatuated, resulting in the precipitation of encrusting scale on hot or rough surfaces, such as pipe and heat exchangers. Permanent hardness is due mainly to calcium and magnesium sulphate and is not affected by heat or pressure change. However if the water is evaporated it will remain and will encrust. HydroFLOW C Range is a technology breakthrough which reliably eradicates limescale problem. The effect has been demonstrated in all installations worldwide. It was introduced to Electrical and Mechanical Services Department of Hong Kong Government in October of 2004 and is widely adopted in hospitals and correctional centres now. |
|||||
|
|||||
| How
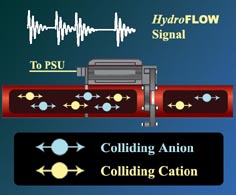
It Works HydroFLOW C Range produces a specific diminished sinusoidal electric field signal with randomly OFF sequence . The length of the OFF sequence allows adjacent ions move together to form clusters. These clusters representing larger dipoles are affected by the electric field during the ON sequence, and are joined together to form localised areas of high concentration. The internal forces generated by these larger clusters result in a contraction and concentration of the attraction forces, this causes the collapse of the clusters into nuclei which are the seed of crystal.
|
 |
||||
The presence of the HydroFLOW C Range electric field throughout the solute will enhance the formation of these large clusters by orientating them, in both the saturated and unsaturated solutions. This process attracts more charged species and stable nuclei are formed. The attraction forces of such nuclei become much greater and as ions diffuse to the nuclei surface, a diffusion layer is formed and ion become incorporated into the crystal lattice. Crystal are formed and grow, again helped by the effect of orientation of the ions by the applied field, and aggregate to form larger crystals. When HydroFLOW C Range is used, the diffusion is enhanced because the ions are orientated by the electric field being applied. For this process to occur throughout the system, the field has to be present throughout the solution, especially closer to the area where the solution will experience changes in temperature or pressure which are responsible for the precipitation of the salts from solution. Small crystals aggregate to form larger crystals that grow at the expense of smaller crystals. The energy and time necessary to orientate and move the charged species together, will vary for different species. Any crystallising system is characterised by the generation of a spectrum of differently sized particles. The electric field applied by HydroFLOW C Range allows for these differences. The following pictures show the impurities in ionic form which is free floating in the solution. When there is a change of temperature or pressure, hard scale as shown is formed. This process is accelerated by a rough surface.
Let's take a look at the difference made by HydroFLOW C Range. Those ions are aligned by the electric field induced into the water and crystallisation is promoted in the solution. As a result existing scale is dissolved into the unsaturated water and no hard limescale is deposited on the surface.
|
|||||
HydroFLOW C Range does not rely on water flow rate and hence it can be applied to any applications where limescale is found. The list of applications is too lengthy to be put here but the followings is the typical devices which we can protect.
Existing scale is normally dissolved. The time taken depends on the volume of water, the flow of water to remove the excess scale crystals, the porosity of the old scale and variation in the temperature and the pressure of the water. In most cases the process is fairly rapid and up to 95% or more of old scales is dissolved within the first three months if blown down or bleed-off of water is in function according to the specification. |
|||||
| Copyright © 2015 PowerTech IPC Company Limited. All rights reserved. | |||||